What Internists Need to Know About Acute Respiratory Distress Syndrome (ARDS)
Written by Maddy Crouch

Key takeaways:
- ARDS is hypoxemic acute respiratory failure due to inflammatory lung injury with bilateral noncardiogenic pulmonary edema.
- ARDS usually has a direct or indirect precipitating event. Aspiration and sepsis are the most common. Risk of ARDS increases with multiple precipitating events.
- The 2012 Berlin Conferences requires that 4 criteria are met for diagnosis of ARDS.
- Patients initially present with symptoms of dyspnea, increased work of breathing, and eventual respiratory fatigue.
- Treat the underlying condition and provide optimal cardiopulmonary support. Give empiric antibiotics if infection is suspected.
As the weather grows colder and people are forced indoors, you know you'll start seeing more patients with respiratory disease. This winter, you can prepare for respiratory season when you get the entire Pulmonary Medicine section of our Core for free.
In this blog, we'll review what you need to know about Acute Respiratory Distress Syndrome (ARDS) for boards and in your practice this respiratory season. This information has been pulled from our 20th Edition Internal Medicine Core.
ARDS Overview | ARDS Diagnosis | ARDS Treatment
Acute Respiratory Distress Syndrome Overview
ARDS is hypoxemic acute respiratory failure due to inflammatory lung injury with bilateral noncardiogenic pulmonary edema.
ARDS usually has a direct or indirect precipitating event. Direct causes include aspiration, pneumonia, and inhalation injuries. Indirect causes are sepsis, pancreatitis, multiple transfusions, and trauma. Aspiration and sepsis are the most common. Risk of ARDS increases with multiple precipitating events.
ARDS lecture by Raj Dasgupta from the Internal Medicine Video Board Review
Diagnosis of ARDS
The 2012 Berlin Conferences requires that all of the following 4 criteria are met for diagnosis of ARDS:
- Onset of respiratory symptoms within one week
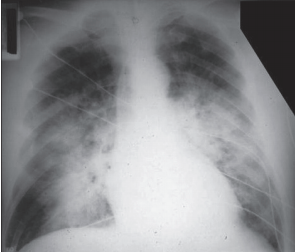
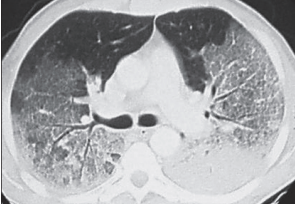
- Chest x-ray or lung CT findings consistent with (noncardiogenic) pulmonary edema—not otherwise explainable (Figure 6-32 and Figure 6-33)
- Increased hydrostatic pressure is ruled out (by echocardiogram if needed) so that the respiratory failure cannot be fully explained by fluid overload or cardiac failure.
- Moderate-to-severe hypoxemia. The severity of hypoxemia is based on the ratio of PaO2 in mmHg to Fi O2 expressed as a decimal (0.21-1.00). In a patient on a ventilator with PEEP ≥ 5 cm H2O:
- Mild ARDS: PaO2/Fi O2 > 200 but ≤ 300
- Moderate ARDS: PaO2/Fi O2 > 100 but ≤ 200
- Severe ARDS: PaO2/Fi O2 ≤ 100
The mnemonic ARDS can be used to recall the Berlin criteria:
- Acute onset
- Restrictive lung mechanics from pulmonary infiltrates
- Diffuse panendothelial inflammatory injury manifested in the lungs—i.e., not due to hydrostatic pressure
- Shunt hypoxemia—The degree of hypoxia defines severity of ARDS.
There is typically a 48–72-hour lag time between the injury and onset of ARDS; it is quicker with neurologic insults and trauma-related lung injury.
Figure 6-32: PA chest—ARDS
Patients initially present with symptoms of dyspnea, increased work of breathing, and eventual respiratory fatigue.
With supportive care, patients improve and enter a proliferative phase, during which their lungs repair and organize. Usually, this is when they can be weaned from ventilator support. A few patients develop mild fibrosis and remain ventilator- and/or oxygen-dependent for a prolonged period. A restrictive pattern may be detectable on pulmonary function tests (PFTs) even after a year. As yet, there is no intervention to effectively prevent ARDS.
The death rate has improved with the use of lung-protective ventilatory support strategies and improved critical care support, but remains high (25–58%). The most common cause of death within the first 3 days after the onset of ARDS is the underlying cause! After 3 days, the most common cause of death is nosocomial pneumonia and sepsis.
Diagnosis of pneumonia in the patient with ARDS is difficult because infiltrates and leukocytosis are common in ARDS. Consider bronchoscopy if the diagnosis is uncertain or if a specific diagnosis is entertained (e.g., cytomegalovirus [CMV], Aspergillus, Nocardia, Pneumocystis jiroveci pneumonia [PJP]) or eosinophilic pneumonia and management will be altered by bronchoscopy results.
Treatment of ARDS
Hand hygiene, isolation precautions, and sterile technique can help prevent nosocomial infections in patients with ARDS.
Treat the underlying condition and provide optimal cardiopulmonary support. Give empiric antibiotics if infection is suspected.
Keep patients slightly hypovolemic, while providing sufficient volume to maintain adequate cardiac output and tissue oxygen delivery. The goal is to prevent shock, lactic acidosis and multiorgan failure.
The ARDS Network FACT trial (2006) demonstrated that conservative fluid replacement (targeting normal central venous pressure [CVP], pulmonary artery occlusion pressure [PAOP], and hemodynamic function) was better than a liberal fluid replacement strategy: less (fluid) is more (beneficial). It led to decreased ICU and ventilator time and decreased organ dysfunction. A 2020 study found early diuretic use independently decreased hospital mortality.
Nutrition should be enteral rather than parenteral, if possible. This reduces the risk of central line-associated bacterial infection, and may prevent the translocation of endotoxin and gram-negative colonic bacteria.
Ventilator Support for ARDS
PEEP stands for positive end-expiratory pressure. On the ventilator, a valve shuts when the patient is near end-expiration, while there is still positive intrathoracic pressure.
ARDS displays shunt physiology. The best way to improve oxygenation is to recruit, or “open,” atelectatic and fluid-filled alveoli. At the same time, you want to avoid barotrauma and oxygen toxicity. In general, you can increase PaO2 by either increasing Fi O2 or increasing PEEP. To avoid oxygen toxicity, try to obtain a PaO2 of ≥ 60 mmHg with Fi O2 < 60% ASAP. To open alveoli, use PEEP.
To avoid ventilator-induced lung injury (VILI), use adequate but not excessive PEEP (i.e., the minimum level of PEEP that allows an adequate mean arterial pressure (MAP) and a safe Fi O2 of 60%).
When managing ventilators, keep in mind the potential for VILI, which arises from overdistended alveoli (from an increased tidal volume [TV] or an increased endinspiratory plateau pressure) and the cyclic opening and closing of atelectatic alveoli (recruitment-derecruitment). An adequate level of PEEP prevents repetitive closure and opening of lung units.
Recommendations from 2000 from the NIH ARDS Network (and reconfirmed with a Cochrane analysis in 2013) indicate better outcomes with low TVs (i.e., < 6 mL/kg) and adequate PEEP.
Previously, TVs in the range of 12–15 mL/kg had been used, but this is too high for many patients with ARDS because their TLC is much smaller than normal. There is a trend to lower TVs in all patients on ventilators, whatever the cause (although there is no strong data to support a benefit, as there is in ARDS). Generally, we start worrying about VILI due to excessive plateau pressures at > 30 cm H2O.
No single ventilator mode has proven better than another for patients with ARDS.
Initial settings:
- Fi O2 = 1, then titrate down as tolerated, keeping PaO2 55-88 mmHg
- TV = Start at 6 mL/kg predicted body weight (PBW). Monitor end-inspiratory plateau pressure! Adjust TV to maintain it at ≤ 30 cm H2O.
- Inspiratory flow = Start at 60 L/minute.
- PEEP: The PEEP is determined using a nomogram of Fi O2 to achieve a PaO2 goal of 55–80 mmHg. For more information, see the NIH NHLBI ARDS Clinical Network Mechanical Ventilation Protocol.
- Lung protective ventilatory support strategy is discussed further later in this topic.
Novel therapies (e.g., granulocyte-macrophage colonystimulating factor [GM-CSF], stem cells, nitric oxide, surfactant, ketoconazole) and empiric neuromuscular blockade have not been demonstrated to improve survival.
A metaanalysis demonstrated a survival benefit from prone positioning, a.k.a. proning. The 2013 PROSEVA trial demonstrated a survival benefit with early initiation of prolonged (16 hours) proning. Issues around proning:
- Data supports increasing the PaO2/Fi O2 ratio with prone positioning, which stabilizes the anterior chest wall, improving physiology and recruitment of previously unused alveolar units.
- The prone position displaces the heart from the left lower lobe and improves lung expansion.
- The prone position can cause pressure necrosis of the face and anterior body surfaces. Other adverse outcomes include extubation, loss of venous catheters, blindness, even fractures. Well-trained ICU staff is paramount for successful prone positioning success.
Permissive hypercapnia: With low tidal volume ventilation (i.e., lung protective ventilation), patients are permitted to maintain a mild respiratory acidosis. This strategy reduces mortality and is the standard of care in patients with ARDS.
The goal is to maintain adequate tissue oxygenation by maintaining the SaO2 > 88%. Do not worry so much about the PaCO2. Hypercapnia with the resultant respiratory acidosis can be corrected by any of the following:
- Increasing the respiratory rate (RR)
- Increasing the TV if the end-inspiratory plateau pressure is low
- Using NaHCO3 Remember: Giving NaHCO3 typically increases CO2 as HCO3 + H+ ↔ H2O + CO2.
Typically, you do not need to give an alkali or increase the minute ventilation, because the kidneys compensate for the respiratory acidosis by retaining HCO3 −. Permissive hypercapnia is also acceptable in severe COPD exacerbation. Again, your treatment target is the SaO2, not the PaCO2.
Permissive hypercapnia is not recommended in patients with intracranial hypertension or hemodynamic instability.
Trials show no proven survival advantage for the following treatments for ARDS: early high-frequency oscillation, surfactant administration, NSAIDs, antiendotoxin therapy, inhaled nitric oxide, and IV N-acetylcysteine (an antioxidant).
To summarize, when using the ventilator in the treatment of ARDS, avoid VILI and oxygen toxicity by doing the following:
- Start the TV at 6 mL/kg of the IBW. Keep plateau pressure ≤ 30 cm H2O.
- Start the Fi O2 at 1. Titrate down as tolerated, keeping the PaO2 55–88 mmHg.
- Use adequate but not excessive PEEP.
- Use permissive hypercapnia if needed.
- Adjust PEEP and Fi O2
Corticosteroids
The pendulum has shifted away from the routine use of steroids to treat ARDS. If steroids are used, start early (first 14 days) in moderate-to-severe ARDS.